Oettgen Laboratory Research | Overview
Research Focus: Mechanisms of Immune Sensitization and Inhibition in Food Allergy
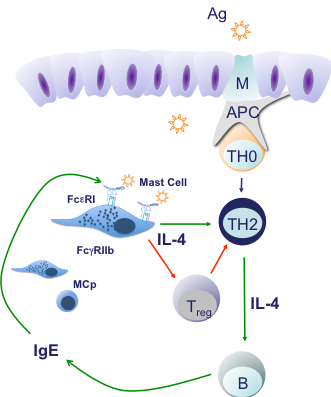
Treatments to prevent or cure food allergy are virtually non-existent. The Oettgen lab uses mouse models and translational studies of food-allergic patients to delineate mechanisms leading to allergic sensitization to ingested antigens as well as regulatory pathways promoting tolerance. Using inherently atopic mice, we have established that IgE-activated mast cells serve not only to release the mediators responsible for anaphylactic reactions but also play a critical regulatory role in adaptive immune responses; suppressing the induction of effective peanut-specific Treg cells and promoting the emergence of pro-allergic Th2 cells. IgE blockade with antibodies or pharmacologic inhibition of FceRI signaling during peanut sensitization in this model led to the emergence of an effective Treg response to peanuts and failure to develop food allergy. Strikingly, inhibition of the IgE:mast cell axis in animals with established allergy was effective in eliminating Th2 responses; inducing Treg and essentially curing food allergy. Thus we hypothesize that IgE antibodies not only mediate acute allergic reactions but also fuel and consolidate Th2 responses to food allergens in the intestinal mucosa.
Using both the mouse model and human studies of oral immunotherapy (OIT) to peanut we have also recently shown that food allergen ingestion leads to the induction of IgG antibodies that signal via the inhibitory IgG receptor, FcyRIIb, on mast cells and basophils to counteract IgE-mediated activation. We believe this inhibitory IgG mechanism is important in both OIT and in the natural resolution of sensitivity in children outgrowing their food allergies. We are currently evaluating the effects of food-specific IgA antibodies in regulating the function of allergic effector cells.
Our current studies are focused on identifying key checkpoints in IgE and IgG responses in food allergy and on developing innovative strategies to target them. We are evaluating how IgE blockade (with the anti-IgE monoclonal antibody, omalizumab) alters allergic sensitization in both food allergy and asthma. The goal of this work is to test whether, like in the mouse food allergy model described above, IgE antibodies amplify emerging Th2 responses and suppress Tregs. We are additionally evaluating whether cell intrinsic differences in basophil sensitivity to activation by IgE and food allergens can account for the wide range of reactions exhibited by individuals with similar levels of allergen-specific IgE to foods like peanut, egg and milk.