For Patients | Overview
What is a research study?
Research is the way doctors and scientists learn about new ways to prevent and treat conditions, and find answers to many questions about health, disease or human behavior. As a participant in a research study, you play a critical role in improving health care and future treatments; however, it is also an important personal decision.
Some participants may have a disease or condition the researchers are studying, while other participants are healthy volunteers. In each research study, there are requirements regarding who can participate. This is called inclusion criteria. There also are reasons why someone might not qualify, known as exclusion criteria.
Being part of a research study is completely voluntary, and you can change your mind at any time.
The research team
Every study has a research team who work together to follow what's called a "protocol" — a detailed plan for the study. The protocol balances the potential benefits and risks to participants, and answer specific research questions.
Research team members include:
- Principal investigator (PI): The PI, who is often a doctor or scientist, is responsible for the entire study. This person plans the study, makes sure activities are done safely and correctly, and decides what each research team member does. The PI supervises all research activities and all of the members of the study team. The PI also makes sure everyone on the team has the proper training.
- Co-Investigators: Many studies have co-investigators — researchers (often doctors or scientists) — who work with the PI. Co-investigators might help the PI design the study or supervise it, but they are not responsible for the entire study.
- Research nurse: A research nurse often has special training and experience. Many studies that involve medical or clinical research have a research nurse on the team who helps carry out the study, focusing on the care and safety of research participants, and is often involved in coordinating daily study activities.
- Research coordinator/research assistant: The research coordinator/research assistant is a person who works with the other research team members to organize and help with daily study tasks.
Other individuals also may help with a research study, such as pharmacists, lab technicians, dieticians who help with participants dietary needs, social workers and office staff.
What are clinical trials?
Clinical trials are medical research studies in which people participate as research volunteers. There are some clinical trials that help researchers understand how a disease or disorder progresses through a person’s life.
Clinical trials are a means of developing treatments, medications, or new approaches for dealing with diseases and conditions.
In a clinical trial, researchers observe the effects an intervention may have on participants. The researcher collects data (information) about how the intervention affects participants’ health. The intervention may be a medical drug or device or procedure; it might also be a behavior change that participants agree to try.
There are several types of clinical research studies, including:
- genetic studies to find the role of genes in different diseases
- prevention studies to test ways to prevent specific diseases
- behavioral studies test how people act in different situations
- physiological studies to increase understanding of how the human body functions
The goal of clinical trials is to determine if these treatment, prevention, and behavior approaches are safe and effective. People take part in clinical trials for many reasons. Healthy volunteers say they take part to help others and to contribute to moving science forward. People with an illness or disease also take part to help others, but also to possibly receive the newest treatment and to have added (or extra) care and attention from the clinical trial staff. Clinical trials offer hope for many people and a chance to help researchers find better treatments for others in the future.
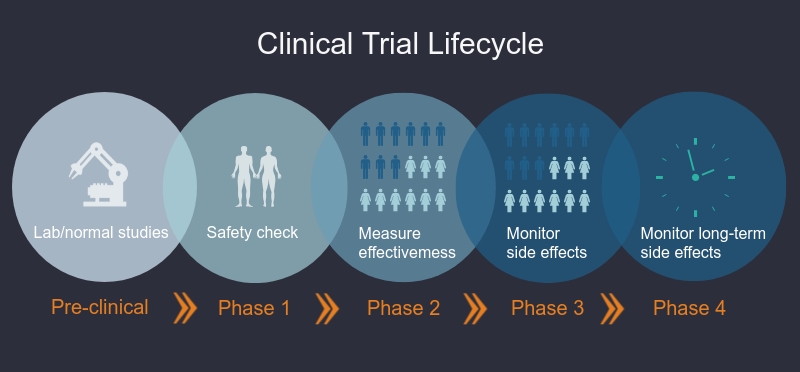
What are the phases of clinical trials?
Clinical trials are done in different phases. In Phase I trials, researchers test an intervention in a small group of healthy volunteers to discover if the intervention is safe, the correct dosage, and if volunteers have any reactions to or side effects from the intervention. In Phase II-Phase IV studies, researchers want to learn more about the safety of the intervention and how well it works in larger groups of participants.
Phase I trials
Researchers test a drug or treatment in a small group of people (20–80) for the first time. The purpose is to study the drug or treatment to learn about safety and identify side effects.
Phase II trials
The new drug or treatment is given to a larger group of people (100–300) to determine its effectiveness and to further study its safety.
Phase III trials
The new drug or treatment is given to large groups of people (1,000–3,000) to confirm its effectiveness, monitor side effects, compare it with standard or similar treatments, and collect information that will allow the new drug or treatment to be used safely.
Phase IV trials
After a drug is approved by the FDA and made available to the public, researchers track its safety in the general population, seeking more information about a drug or treatment’s benefits, and optimal use.
How do clinical trials work?
For most clinical trials a participant is assigned to either an intervention or a control group. Intervention group participants receive the intervention that is being tested.Control group participants do not receive the intervention. Instead they might receive the standard treatment for the disease or condition, or they might receive a placebo, which looks or feels like the intervention, but it is not an active medicine or treatment. The control group helps the researchers understand the effects of the experimental intervention.
Your group assignment often happens by chance. If you take part in a clinical trial, you might not get the experimental treatment that is being tested. In some cases, you won’t know the group to which you are assigned.
Potential benefits and risks of clinical trials
There are benefits and risks for research participants. Being part of a clinical trial allows the participants to have access to experimental treatments that are not yet available to the public, while also helping others by contributing to the development of a new medical treatment or procedure. Additional benefits include:
- helping others by contributing to knowledge about new treatments or procedures
- gaining access to new research treatments before they are widely available
- receiving regular and careful medical attention for a research team that includes doctors and other health care professionals
Risks for research participants include:
- receiving an experimental treatment when its effects are not yet fully understood
- unpleasant or even serious side effects from some tests and procedures
- minor discomfort
- complications that require medical attention
The specific risks associated with a research protocol are described in detail in the informed consent document, which participants are asked to consider and sign before participating in research. Also, a member of the research team will explain the study and answer any questions about the study. Before deciding to participate, it's important to carefully consider risks and possible benefits.
Where do I find clinical trials?
One good way to find out if there are any clinical trials that might help you is to ask your doctor. Other sources of information include:
- FDA Clinical Trials Search. Search a database of Federally and privately supported studies available through clinicaltrials.gov. Learn about each trial’s purpose, who can participate, locations, and who to contact for more information.
- Clinicaltrials.gov. Conduct more advanced searches
- National Cancer Institute or call 1–800–4–CANCER (1–800–422–6237). Learn about clinical trials for people with cancer.
Are clinical trials safe?
FDA works to protect participants in clinical trials and to ensure that people have reliable information before deciding whether to join a clinical trial. The Federal government has regulations and guidelines for clinical research to protect participants from unreasonable risks. Although efforts are made to control the risks to participants, some may be unavoidable because we are still learning more about the medical treatments in the study.
The government requires researchers to give prospective participants complete and accurate information about what will happen during the trial. Before joining a particular study, you will be given an informed consent document that describes your rights as a participant, as well as details about the study, including potential risks. Signing it indicates that you understand that the trial is research and that you may leave at any time. The informed consent is part of the process that makes sure you understand the known risks associated with the study.
You and your doctor are a team in your care. This material is for informational purposes only and it is not intended to be a substitute for medical advice. Talk to your doctor to decide the right course of treatment for you.
