About the Study
What is the goal of the APEX study?
We are doing a study to learn more about how adolescents feel after they have surgery and take medicine for pain. We want to know how their lives are before and after the surgery and how it affects the pain and medicine they need. We will use this information to help doctors take care of adolescents who have surgery in the future.
Who can participate?
Adolescents and their parents are invited to participate in this research study if the adolescent is between 12-17 and scheduled for eligible surgery at a participating hospital. Study staff will ask potential participants a few questions to see if they are eligible to participate. About 10,000 adolescents and their parent/caregiver from these hospitals will participate in this study (20,000 people total).
Why should I participate the APEX study?
Results from this study will help young people undergoing surgery in the future by improving decision-making about pain management and recovery following surgery. Being in this research study may not help participants right now. When we finish the research, we hope that we will know more about pain management in adolescent surgical patients. This may help other young people in the future. Adolescents will receive electronic gift cards for participating in the study.
How is this program funded?
A grant from the National Institute on Drug Abuse (NIDA) will provide funding for this study. It is a multi-center study conducted at Boston Children’s Hospital. A total of 5 hospitals will participate nationwide. Dr. Sharon Levy and Dr. Joseph Cravero are the Co-Lead Investigators.
Participation
What will be expected of me if I participate in the APEX study?
Potentially eligible patients and families will be contacted by study staff. They will receive information about the study and, if interested, complete an anonymous eligibility screener. If eligible, adolescents and caregivers will complete a consent and assent session with a staff member to officially enroll in the study. Once enrolled, the adolescent and a parent/caregiver will be asked to complete surveys before the surgery, and then 4 weeks, 3 months, 6 months, and about one year after surgery. Each of these surveys takes 10-20 minutes. We will also ask adolescents 2-3 daily brief questions about pain and medication use for the first 10 days after surgery.
We will also gather details about the surgery, how the adolescent felt after the surgery, and if they had to go back to the hospital within 1 year after surgery from the adolescent’s electronic medical record (EMR).
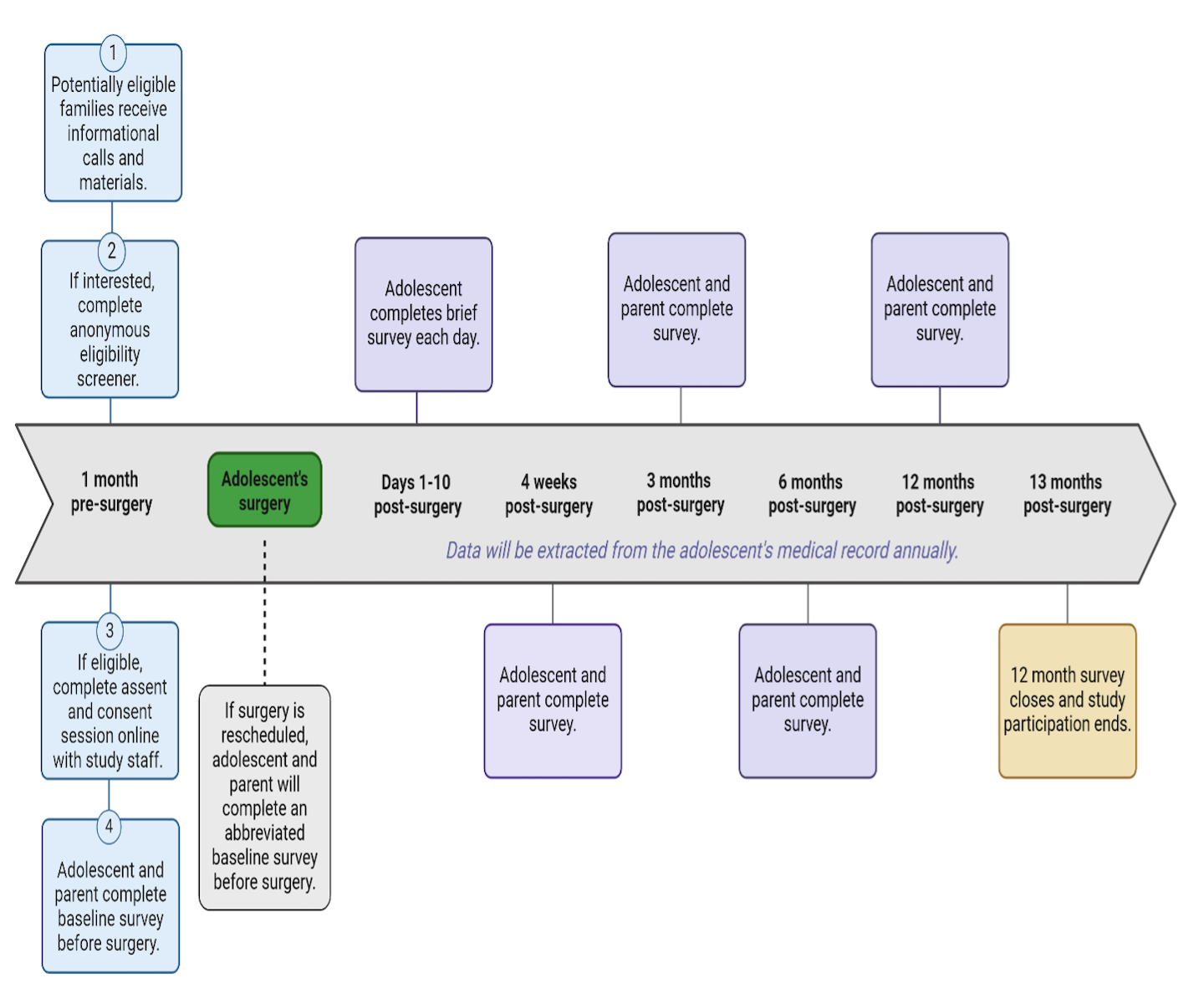
What’s the total time commitment?
The first steps to participate in this study is an informational call, eligibility screen, and assent and consent session. This will take 20-30 minutes.
If participants decide to enroll in the study, they will participate for up to 14 months, completing electronic surveys at various timepoints. We anticipate the first and last surveys will take about 20 minutes while the others will take about 10 minutes. For the first ten days after surgery, adolescents will be asked to answer two or three brief questions each day, which will take about two minutes. The estimated total time to complete surveys is seventy minutes over 14 months.
I’m traveling to another hospital for my surgery. Can I still participate?
Adolescents who are undergoing eligible surgery at a participating hospital are potentially eligible for this study. Even if this hospital is not the family’s regular hospital and they are traveling to receive the surgery, they can participate in the study.
Do I need a computer?
Most participants will complete the electronic surveys on a computer or smartphone. If your family does not have access to this technology, each survey can be completed during a phone call with a study staff member. The informed consent and assent process can be completed over the phone, video call, or in-person. If technology poses a challenge for participating in this study, families are encouraged to let the study staff know.
Will my health information be secure? How will you ensure privacy?
The information we collect for this study may include personal information, such as name, address, birth date, and information from the adolescent’s medical record. Participants’ health information is protected by a law called the Health Information Portability and Accountability act (HIPAA). In general, anyone who is involved in this research, including those funding and regulating the study, may see the data, including information about you. The results of this research may be published in a medical book or journal or be used for teaching purposes. However, only aggregate data will be published no names or identifying information will be used without specific permission from the participants.
Will my doctor see my answers?
Adolescents’ physicians will not see their survey answers. If during participation in this research there is reason to believe that the adolescent is at risk of hurting themselves or others, the research team is required by law and Boston Children’s Hospital policy to act on this suspicion. This may include notifying physicians at the hospital. In these cases, confidentiality may not be ensured.
Who can I contact if I have more questions?
Visit the contact us page to find the contact information about your site. For general information about this research, contact the Boston Children’s Hospital APEX team.
Sobre el estudio
¿Cuál es el objetivo del estudio APEX?
Estamos haciendo un estudio para obtener más información sobre cómo se sienten los adolescentes después de una operación y tomar medicamentos para el dolor. Queremos saber cómo son sus vidas antes y después de la operación y cómo afecta el dolor y los medicamentos que necesitan. Usaremos esta información para ayudar a los médicos a cuidar de los adolescentes que tengan una operación en el futuro.
¿Quién puede participar?
Se invita a los adolescentes y a sus padres a participar en este estudio de investigación si el adolescente tiene entre 12-17 años y está programado para una operación elegible en un hospital participante. El personal del estudio hará algunas preguntas a los posibles participantes para saber si son elegibles para participar. Unos 10,000 adolescentes y sus padres/cuidadores de estos hospitales participarán en este estudio (20,000 personas en total).
¿Por qué debería participar en el estudio APEX?
Los resultados de este estudio ayudarán a los jóvenes que se van a operar en el futuro, ya que mejorarán la toma de decisiones sobre el manejo del dolor y la recuperación después de la operación. Estar en este estudio de investigación puede no ayudar a los participantes en este momento. Cuando terminemos la investigación, esperamos saber más sobre el manejo del dolor en pacientes quirúrgicos adolescentes. Esto puede ayudar a otros jóvenes en el futuro. Los adolescentes recibirán tarjetas de regalo electrónicas por participar en el estudio.
¿Cómo se financia este programa?
Un subsidio del Instituto Nacional sobre el Abuso de Drogas (NIDA) dará el financiamiento para este estudio. Es un estudio multicéntrico que se hará en Boston Children’s Hospital. Un total de 5 hospitales participarán en todo el país. La Dr. Sharon Levy y el Dr. Joseph Cravero son los coinvestigadores principales.
Participación
¿Qué se espera de mí si participo en el estudio APEX?
El personal del estudio se comunicará con los pacientes potencialmente elegibles
y sus familias. Recibirán información sobre el estudio y, si están interesados, completarán una evaluación anónima de elegibilidad. Si son elegibles, los adolescentes y los cuidadores completarán una sesión de consentimiento y aprobación con un miembro del personal para inscribirse oficialmente en el estudio. Una vez inscritos, pediremos al adolescente y a un padre/cuidador que completen encuestas antes de la operación, y luego 4 semanas, 3 meses, 6 meses y aproximadamente un año después de la operación. Cada una de estas encuestas dura entre 10-20 minutos. También haremos a los adolescentes 2-3 preguntas breves diarias sobre el dolor y el uso de medicamentos durante los 10 primeros días después de la operación.
También recopilaremos información sobre la operación, cómo se sintió el adolescente al terminar el procedimiento y si tuvo que volver al hospital en menos de 1 año después de la operación según el expediente médico electrónico (EMR) del adolescente.
¿Cuál es el compromiso de tiempo total?
Los primeros pasos para participar en este estudio son una llamada para informar, una evaluación de elegibilidad y una sesión de aprobación y consentimiento. Tomará entre 20 y 30 minutos.
Si los participantes deciden inscribirse en el estudio, participarán hasta 14 meses, completando encuestas electrónicas en diferentes momentos. Prevemos que la primera y la última encuesta le tomarán unos 20 minutos, mientras que las demás tardarán unos 10 minutos. Durante los diez primeros días después de la operación, se pedirá a los adolescentes que respondan a dos o tres preguntas breves cada día, que tomarán unos dos minutos. El tiempo total que se calcula para completar las encuestas es de setenta minutos a lo largo de 14 meses.
Voy a viajar a otro hospital para mi operación. ¿Todavía puedo participar?
Los adolescentes que se hagan una operación elegible en un hospital participante son potencialmente elegibles para este estudio. Incluso si este hospital no es el habitual de la familia y viajan para hacer la operación, pueden participar en el estudio.
¿Necesito una computadora?
La mayoría de los participantes completarán las encuestas electrónicas en una computadora o un teléfono inteligente. Si su familia no tiene acceso a esta tecnología, cada encuesta puede completarse durante una llamada por teléfono con un miembro del personal del estudio. El proceso de consentimiento informado y aprobación puede completarse por teléfono, videollamada o en persona. Si la tecnología supone un reto para participar en este estudio, se anima a las familias a que se lo comuniquen al personal del estudio.
¿Estará segura mi información médica? ¿Cómo se asegurará la privacidad?
La información que recopilamos para este estudio puede incluir datos personales, como el nombre, la dirección, la fecha de nacimiento e información del expediente médico del adolescente. La información médica de los participantes está protegida
por una ley llamada Ley de Portabilidad y Responsabilidad del Seguro Médico
(Health Insurance Portability and Accountability, HIPAA). En general, cualquier persona que participe en esta investigación, incluyendo a los que dan financiamiento y regulan el estudio, pueden ver los datos, incluyendo información sobre usted. Los resultados de esta investigación pueden publicarse en un libro o revista de medicina o pueden usarse para fines de enseñanza. Sin embargo, únicamente se publicarán datos globales, no se usarán nombres ni información de identificación sin el permiso específico de
los participantes.
¿Mi médico verá mis respuestas?
Los médicos de los adolescentes no verán sus respuestas a la encuesta. Si durante la participación en esta investigación hay motivos para creer que el adolescente corre el riesgo de hacerse daño o hacerles daño a otros, el equipo de investigación está obligado por ley y por la política de Boston Children’s Hospital a actuar ante esta sospecha. Esto puede incluir avisar a los médicos del hospital. En estos casos, la confidencialidad puede no estar asegurada.
¿Con quién puedo comunicarme si tengo más preguntas?
Visite la página contact us (comuníquese con nosotros) para encontrar la información de contacto sobre su lugar. Para obtener información general sobre esta investigación, comuníquese con el equipo APEX de Boston Children’s Hospital.
